Is Fuel Dilution Just a Hybrid Thing?
A lot of worries pass through our lab each day. Some are warranted, like when knocking or coolant loss ends up manifesting in high bearing wear or coolant contamination in testing. But
just as many worries stem from common misconceptions and when that happens, we’re happy to assuage these concerns. Occasionally, though, a trend will coalesce in our data, seeming to
confirm a common assumption. Today we’re delving into the assumption that hybrid engines are more prone to fuel dilution than conventional ones, and we’ll try to determine whether or not that
should keep you up at night.
A Grain of Truth
A lot of hybrid owners dismiss fuel dilution in their samples, deeming it inevitable for motors working only part of the time. Others are less comfortable with repeated high fuel readings, and
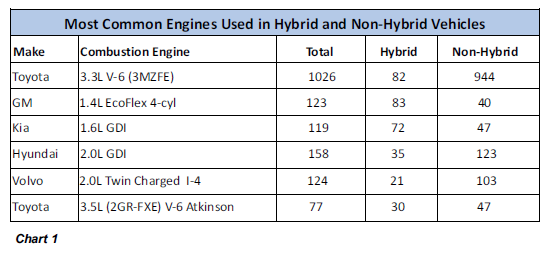
seek advice on how to make it go away once and for all. To address these questions, we started by rounding up six engine models from various manufacturers. These were models used in both
hybrid and conventional vehicles, which also had relatively large sample pools in our database. A breakdown of the number of hybrid and conventional vehicles running these models is shown
in Chart 1.
We then recorded how many samples in each group contained 2.0% or more fuel. Anything less than 2.0% we consider fairly benign, but beyond that threshold, we start to wonder about fuel
system trouble. These results are shown in Chart 2.
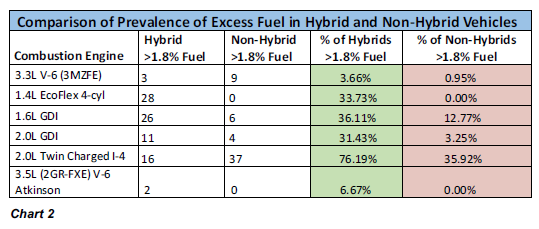
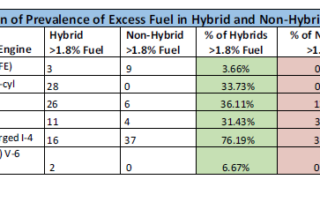
Some engines ended up with only a slightly higher incidence of excess fuel in their hybrid models, while for others the disparity is quite significant. But on average 31% of the hybrids
contained excess fuel, with only 18% of the non-hybrids, seeming to confirm that hybrid engines are more prone to fuel dilution. Which leads us to the question – what’s so bad about fuel getting
into the oil?
Why Fuel Dilution Matters
When I brought my findings to Blackstone president Ryan Stark, he shared his own worst-case-scenario. Picture 1 and Picture 2 show a piston from his ’86 GMC Jimmy, clearly in pretty bad shape. Ryan had been dealing with excess fuel for a long while, which started to gum up his oil control rings. This led to the engine burning more oil and the ash leftover from the burning oil left deposits in the combustion chamber. Over time, these deposits got worse and the engine started to suffer from both pre-ignition and detonation. Eventually, the detonation caused a hole to form on the piston, pressurizing the crankcase, and blowing all the oil out the engine. Luckily, he noticed the oil billowing out of the back of the vehicle and the low oil pressure light on the dashboard and was able to pull over to the side of the road and stop the engine before oil starvation caused it to seize up.


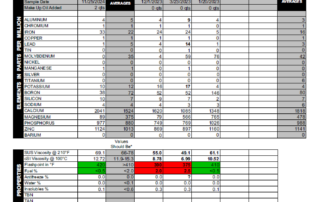
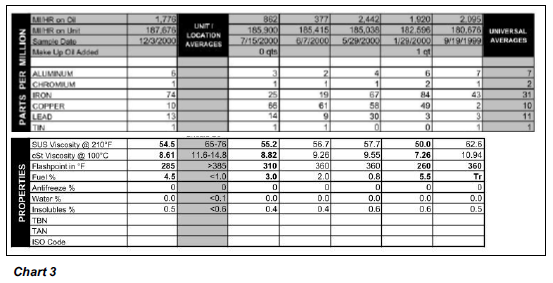
Anyone would want to avoid a fate like this for their engine, so if you find persistent fuel dilution hard to accept, it really only makes sense to try and keep an eye on things using oil analysis. While we no longer have the oil reports from when this problem occurred back in 1996, we do have the last six samples from before Ryan sold this vehicle and you can see the results below (Chart 3). Ryan fixed the piston, yet the engine still had a persistent fuel system problem and the engine likely would have suffered the same fate if the problem was left unchecked. Ryan’s Jimmy was an extreme case. Modern engines are equipped to detect detonation and automatically try to fix the problem by adjusting the timing and fuel mixture. Still, if excess fuel is present, deposits can accumulate over time, but because they line the combustion chamber and form in the ring lands of the pistons, they don’t necessarily show up as wear in the oil. This is why we suggest watching the oil level on your dipstick. If your engine suddenly starts to consume a lot of oil, then it’s possible the oil control rings are no longer working like they should and other engine problems can’t develop. More often than not, poor wear doesn’t go hand-in-hand with excess fuel. A lot of times the fuel actually dilutes metals to such an extent that wear metals actually tend to read well below average, giving you a false sense of security.
After many years of testing oils we are constantly impressed by the resiliency of engines. We don’t live in a perfect world, and fortunately, we don’t have to. At least for a short while, most
engines can withstand some contamination or other mishaps, provided they are addressed promptly enough. That brings us to a couple potential mitigating factors, when it comes to
chronic fuel dilution in hybrid engines specifically.
Hybrids and Fuel Dilution
The combustion engines within hybrid vehicles experience operational factors that traditional vehicles do not. Most notably, they’re continually turn on and off for greatest fuel efficiency. A
hybrid vehicle being used primarily at lower speeds will rely mostly on its electric motor, which is also favored during acceleration. At higher speeds or during prolonged acceleration,
when more power is required, the combustion engine will kick on. This makes for a situation where it’s tough to know exactly how much the combustion engine has been used during a
particular oil run.
It also seems likely that this sporadic use is a major factor in the prevalence of fuel in hybrid engine samples. Most combustion engines run rich during start up, leading to small amounts of
fuel sneaking past the rings. Whatever ends up in the oil would typically evaporate with sustained engine temps of 212 degrees F. There are a few reasons why this might not happen,
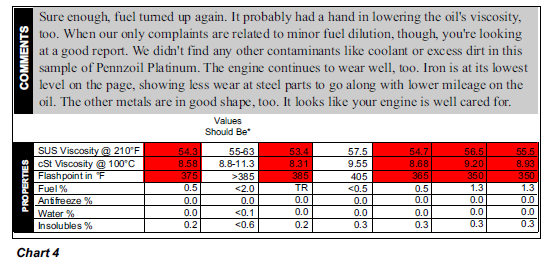
however. Chart 4 is an example of a conventional engine that routinely had small amounts of fuel in its oil, likely due to stop-and-go city traffic. For a hybrid, though, every time the electric
motor kicks on, the combustion engine gets an opportunity to cool back down, leaving any fuel left to accumulate in the oil, rather than cooking out.
Of course, in theory, less use on the engine also means less chance for fuel-related deposits to form. Could the mix of factors impacting hybrids simply balance one another out? From here,
we wanted to answer the burning question: do hybrid engines experience more failures we could associate with chronic fuel dilution? This can be a tricky question to answer directly,
mainly because it relies on customer feedback. Once an engine has failed, it’s not everyone’s instinct to update us on what happened. Then again, some customers certainly do update, and we’ve yet to hear of a scourge of failed hybrid engines for any reason, much less fuel alone. Still, we wanted to see what the hard data had to say about it.
So … is fuel a problem or not?
To answer this question, we selected Kia’s 1.6L GDI engine for a closer look. Of the engines shown in Charts 1 and 2, it had the most representative average difference in fuel incidence
between hybrids and non-hybrids. It also had a fairly even amount of hybrid and non-hybrid samples in our system. We then counted samples that had one or more significantly elevated
wear metal. Of the 119 samples, 64 fit these parameters…yet a great majority were simply going through wear-in. Once we eliminated those, we were left with 12 high-wear samples. When we
further narrowed to samples with excess fuel, and separated hybrids from non-hybrids, the numbers became vanishingly small: 1 for hybrids and 3 for non-hybrids.
So, there does not seem to be a correlation between hybrids, fuel, and excess wear, much less premature engine failures. Regardless, it’s obviously better to have less (if any) fuel in your
engine oil; the less contamination, the more the oil is going to function as designed and tested by the manufacturer. So how can we accurately judge the risk, and mitigate fuel dilution, if noted?
Above all, it’s important to monitor the oil level at the dipstick – if fuel dilution is noticeably displacing the oil level, it’s worth inspecting the fuel system, no matter the reason. It’s also worth
making note if your engine is starting to consume oil, since it can be a sign that the oil control rings are no longer working correctly, which could lead to problems with your catalytic
converter as well as pre-ignition and detonation issues. Hybrid vehicle or not, any combustion engine can develop acute fuel system issues that are worth addressing promptly.
But as for the smaller, nuisance amounts of fuel dilution? It never hurts to work the engine a bit harder every now and again, to generate the heat it takes for fuel to evaporate—for example,
hopping on the highway for 15 minutes or so, if that’s not a normal part of your routine. More frequent oil changes can also help to flush out any fuel that’s mixing with the oil, before it can
accumulate to problematic levels. And of course, there’s always oil analysis! We’re happy to help you determine whether fuel is indeed excessive in your engine oil, and help assess what—if
any—action to take if so. Free sample kits are available at: www.blackstone-labs.com/free-testkits.
The Fuel Experiment
When I first started at Blackstone, one of the contaminants that intrigued me the most was fuel. I guess I don’t know why finding fuel in people’s oil surprised me. Maybe I thought fuel and oil were separated by a giant wall somewhere in the engine. Or maybe (probably) I really didn’t understand engines very well to begin with and that only served to fuel (ha!) my interest in the contaminant.
We test for fuel using the Cleveland Open Cup method. Basically, we record the temperature at which the vapors from the oil ignite. All oils have a specification for what the flashpoint should be. When it’s lower than that, it’s because a contaminant is present. About 98% of the time, that contaminant is fuel (sometimes a solvent or refrigerant will lower a flashpoint, but rarely in gas or diesel engines). Basically, the lower the flashpoint, the more fuel you’ve got.
We can accurately measure fuel down to less than 0.5%, so that’s the lowest fuel measurement you’ll see on your report. The upper limit of what we can accurately read is 10.0%. If you’ve got more fuel than 10.0%, you’ve got bigger problems to worry about than the actual quantity of fuel in the oil.
When the opportunity came up to write an article for the newsletter, I readily accepted and already knew I wanted to write about fuel. In fact, I was not just going to write about it–I was going to get to the bottom of it. I was going to discover what causes fuel dilution and what causes fuel to disappear.
The plan
The guinea pig was my trusty Kia Optima (2.4L, 4 cylinder). I use my Kia mostly as a daily driver, traveling about seven miles to and from work each way. I love to travel and occasionally I get in a trip to Wisconsin or Iowa. By the time I started my quest to debunk fuel, I’d done a few samples with my Kia and only a trace of fuel had ever turned up so I didn’t have any known fuel system problems to contend with.
I decided to take the highway route home every day to ensure that every day I would cook out any extra fuel that was present in my oil. My 40-minute drive consisted of some city streets with a few stoplights at the beginning and end of my trip, and mostly sustained highway speeds through the middle of my trip.
Start your engines
The first thing I wanted to test was how much fuel entered the oil simply from starting the engine. Many people believe that starting an engine is one of the most taxing and wear-producing events throughout the engine’s life. To make that process easier, engines tend to start slightly rich (more fuel, less air). So, I set out to find out exactly how much fuel my car dumps into the oil upon startup.
After letting the engine sit all night, I took a pre-experiment test sample (to ensure no fuel was present) then I started my engine one, two, and three times, sampling after each event. The results were surprising. So surprising, in fact, that I re-ran this test two more times to make sure my results were correct.

The pre-experiment test sample revealed a flashpoint of 360ºF (fuel at <0.5%). Okay, good. No measurable fuel was present, which is exactly what I was hoping for since I’d taken the highway route home the night prior.
After one engine start, the flashpoint read 385ºF. Wait a minute. That’s higher than the pre-sample, so there’s definitely still no measurable fuel present. Okay, maybe that was just an anomaly.
I started the engine again (for a total of two engine starts in a row). The flashpoint measured 380ºF. That meant the flashpoint was heading in the expected direction (lower flashpoint = more fuel), but still, the flashpoint wasn’t low enough to show any significant fuel.
After the third start in a row, the flashpoint read 375ºF, which was again lower (and heading in the expected direction), but not low enough to show any measurable fuel. So all four of my samples from that morning had the same fuel measurement: <0.5%.
I was stumped. I was so certain I’d have some fuel in my oil! So I ran the test again and the same thing happened: no measurable fuel present in any of the samples. I re-ran the same exact test once again, and once again got similar results.
I decided perhaps my Kia was just very good about keeping fuel out of the oil, so I took my husband’s car with a supercharged 2.0L Ecotec engine for a day and tried the same test. The results? Same thing: no fuel, with slight fluctuations in the flashpoint.
Then I had an “a-ha!” moment. Maybe the fuel just wasn’t getting a chance to seep down past the rings into the oil. I had been sampling immediately after starting, so maybe that’s why no fuel showed up. So I ran the test again, only this time I let my engine sit overnight after the three starts.

In the pre-experiment test, the flashpoint measured 370ºF, showing <0.5% fuel. I started my engine three times and immediately after the third start, the flashpoint measured 360ºF, which was lower but still not low enough to show any measurable fuel.
Then I let the engine sit all night and sampled before work in the morning. That test revealed a flashpoint of 365ºF; still no measurable fuel. By this point I wanted to find a brick wall to repeatedly press against my forehead in a semi-violent manner. I was frustrated, confused, and worried that I’d have nothing to write about.
I suppose if we wanted to get into semantics, we could talk about the slight differences in flashpoints as showing some fuel, though it takes a 20ºF drop in flashpoint (for gasoline engines) to show 1.0% fuel dilution. So that means the 5ºF drops in flashpoint I’d noticed likely show just 0.25% fuel.
Does starting cause fuel? Perhaps. I did find slight dips in the flashpoint, though as I’ve mentioned, 5ºF isn’t enough to show any serious contamination. Maybe four starts would have given me enough of a drop in flashpoint to get a decent amount of fuel in the oil, but really, who starts their engine four times in a row? Honestly, who starts their engine even three times in a row on a regular basis? I wanted these to be relatively real-world scenarios, so I couldn’t justify four starts in a row, and I figured three was pushing it.
Idling
I couldn’t get any serious fuel to appear in my oil from starting the engine, so I figured I’d try idling. For a week, I drove the same highway route home, let my engine sit all night, then in the morning I’d take a pre-experiment sample (to confirm no fuel dilution was present to begin with), and then I’d sample after a certain number of minutes of idling.
I tried a five-minute idle; no measurable fuel. The next morning, I idled for ten minutes and this time I had more success: 1.0% fuel had accumulated in the oil. You know, for someone trying to get fuel contamination in my oil, 1.0% isn’t an impressive amount, but it’s more than I’d gotten before. I figured I was on to something with the idling, so I tried 15 minutes the next morning, but much to my dismay only 0.5% fuel turned up. After 20 minutes of idling, still only 0.5% fuel.
Does idling cause fuel dilution? It would seem so, except that there’s a cut-off point in there somewhere. This is just hypothetical, but maybe after ten minutes the engine heats up enough that it either starts cooking off the excess fuel dilution or it just stops pumping in extra fuel. I’m not even sure one of those is the answer, but it’s the best guess I can come up with.
Shopping for science
With my newsletter article deadline quickly approaching, I had to come up with one last-ditch effort to get a bunch of fuel in my oil. Think of this as Custer’s Last Stand (except with less bloodshed).
For one afternoon, I vowed to do everything “wrong” in order to get as much fuel as possible in my oil. I was going to run a bunch of errands, idle my engine excessively, make frequent starts and short trips. The best way I could think to do this (without just circling around my block several times in one afternoon) was in one, massive shopping trip.
I did about 40 minutes of highway driving Saturday evening then let my Kia sit all night. Sunday after church (we took the other car to ensure the consistency of my results), I went on my scientific shopping trip.
Here’s the summary of my trip. I spent a total of about six hours shopping Sunday afternoon. In those five hours, I started my engine seven times, idled at the ATM for about 2 minutes and traveled a grand total of 6.4 miles. The longest drive was from my last stop to home, which was about 2.5 miles.
I spent a fair amount of time at each stop in hopes that my engine would stay relatively cool (so as to not burn off fuel). When all was said and done, I left my engine sit overnight and sampled in the morning before work. The flashpoint read 375 ºF: <0.5% fuel.
Usually, I’m fairly good at things when I put my mind to it, but when it comes to getting fuel dilution in my engine oil, I failed. Then again, if you’re going to fail at anything, this is a good thing to fail at.
What happened?
So why couldn’t I get any serious fuel dilution? I have a couple of ideas. First, I think my Kia is just too smart. It has an on-board computer that senses things like ambient temperature, engine temperature, and elemental composition of the exhaust gas, and it uses these things to calculate the exact amount of fuel it needs to operate most efficiently. So my engine never puts in more fuel than it needs, and therefore that fuel doesn’t end up in my oil.
Second, I think ambient temperature probably has a lot to do with it. I did most of my testing in May and June in temperatures were almost always above 70ºF. We tend to see more fuel in the winter months, and I suspect that if I’d done my testing in the winter, I might have had different results. I’ve heard that on a cold day, fuel from the air/fuel mixture will condense on the cylinder walls almost instantaneously. Those beads of fuel will sit on the cylinder walls for a brief moment until the piston rings scrape that fuel down into the oil. Maybe I’d get more fuel in my oil in winter, but I’m not sure I’m willing to do these experiments in the dead of winter in the name of science. If I do, you’ll hear from me again and I’ll let you know what I find out.
Does the fact that I couldn’t get any fuel in the oil mean that idling, city driving, and frequent starts do NOT cause fuel dilution? We don’t think so. In some cases these things can cause fuel contamination, especially in carbureted engines.
 We saw it with our own eyes several years ago, when an intern did a similar experiment out in the parking lot. He took a sample from his 1978 Ford pickup truck when it was cold, and that oil had no fuel in it. Then he started the engine and took another sample right away, and presto! Fuel contamination at 1.3%. So start-up can indeed cause fuel to enter into the oil, but newer engines may be better at avoiding excessive contamination.
We saw it with our own eyes several years ago, when an intern did a similar experiment out in the parking lot. He took a sample from his 1978 Ford pickup truck when it was cold, and that oil had no fuel in it. Then he started the engine and took another sample right away, and presto! Fuel contamination at 1.3%. So start-up can indeed cause fuel to enter into the oil, but newer engines may be better at avoiding excessive contamination.
Since fuel often comes and goes, we still believe operational factors are likely sources for fuel dilution, though perhaps injector problems are responsible for more of the fuel we see in new engines than we originally thought.
Now here’s the big question: if fuel is present at 2.0% in your sample, does that mean you have a problem? Not necessarily. Just because my Kia didn’t produce fuel dilution doesn’t mean your Honda, Ford, GM, Volvo, or other engine won’t. Every engine operates a little differently and uses a different calculation to figure out how much fuel to spray into the cylinders. Some engines tend to run a little richer for some reason or another.
Of course, if your engine doesn’t have an on-board computer, you won’t have the opportunity to benefit from its fuel-reducing powers, so fuel may be more prevalent in your samples. It should be noted that I didn’t have the opportunity to test a carbureted engine or a diesel engine, which would almost certainly render different results. So I can’t say what’s normal for those types of systems.
So how do you know if fuel is a problem? There isn’t a one-size-fits-all answer. Whether or not fuel is a problem depends on your circumstances. Some engines will always see some fuel dilution because of the operation they see or the type of engine they are. Turbo and supercharged engines, for example, have higher compression, which means more blow-by, and we sometimes see that raw fuel blowing past the rings. In small amounts, that can be fine. In larger amounts, it’s probably not.
If you start to notice that fuel is increasing wear or diluting the additives in your oil, that can be a sign that fuel’s a problem. If you notice increased wear and lingering fuel dilution, it may be time to get the fuel issue taken care of. Finally, if you notice your engine is “making oil” (the oil level seems to be rising on the dipstick), you might have a fuel system problem. I can tell you this: if you have a Kia 2.4L 4-cylinder engine and you find fuel at more than 1.0%, you may have a problem. You might also have an e-mail in your inbox from me asking you for pointers.
Fuel in Diesels
For our last newsletter, we did an experiment where we actually tried to get fuel dilution to show up in the oil. Amanda’s Kia was our guinea pig, and she tried hard to get some fuel to show up but had very little success. She tried idling for ten minutes and she tried lots of city driving, but could hardly get anything more than a trace or so. Maybe that’s just a testament to Kia and their fuel system engineering, or maybe she was just unlucky. It’s hard to say. However, fuel dilution does show up for a lot of our customers and after the last newsletter, we received some e-mails asking for more information about fuel, especially in diesel engines with possible fuel dilution problems.
A little history
Diesel engines started showing up in pickup trucks back in the 1980s and while those engines didn’t particularly wear well, fuel dilution wasn’t really a big issue.
In the 1990s, these engines really started coming into their own. Wear metals improved and the oil changes started getting longer and longer. Ford started using the Navistar 7.3L Power Stroke and Dodge used the Cummins 6BT 5.9L, and both were excellent engines. They produced a lot of power and left very little metal in the oil to show for it.
GM used the Detroit Diesel 6.5L, and while that was a good engine and a lot of them are still on the road today, it tended to make a lot more metal than its competitors. It wasn’t until GM started the Isuzu 6.6L Duramax that it really had a world- class diesel that was every bit as good as what Ford and Dodge were using.
With this new generation of engines, we started seeing people run 5,000-mile oil changes regularly, where the old standard was just 3,000 miles. And oil changes have gotten longer and longer since.
These days it’s not uncommon at all to see those engines running 10,000 miles on the oil without any special oil filtration set-up. Of course, a lot of that is dictated by the type of use they see. This was also the carefree days before emission controls starting becoming mandatory.
For some of you, the words emission controls may make you turn away in disgust and I’ll admit, on my own truck engine (a gasoline powered GM 350), the emission controls haven’t gotten the attention the rest of the engine has. But really the idea isn’t really all that bad.
Piston powered aircraft engines don’t have any emission controls on them, but those engines are plagued by rust and corrosion because condensation from the air is allowed to enter through the breather. Modern gasoline and diesel engines don’t have that problem because their crankcases are sealed to the elements and that keeps corrosion to a bare minimum. It’s also one of the reasons you don’t really need to change your oil on a time basis anymore. We get a lot of questions about if an oil will last a year or not and the answer is almost always yes, because very little corrosion builds up on these engines.
Of course, gasoline-powered engines have had emission control systems on them since the 1970s and that means the engine designers have had a lot of time to get it right. When emission controls started appearing on diesel engines in 2005 and 2006, there were a lot of growing pains with that introduction. Couple that with the fact that competition brought about the need for more and more power, and now we started seeing changes in the oil samples, mainly at fuel dilution.
We first started seeing a lot of fuel when Navistar came out with the 6.0L Power Stroke in 2003. Those engines almost always had a lot of fuel in the oil, especially when they were new¾and when I talk about a lot, I mean 4% and 5%.
We weren’t sure exactly what cased this, but it was showing up in almost every sample we saw and this presented a problem for us because we had always considered 2.0% to be an “action” level of fuel. So what do you do when every engine starts showing more than 2.0% fuel? Do you start sending every owner back to the dealer saying there’s a problem? And what do you do if you see a lot of fuel dilution, but wear metals continue to look good?
So the 6.0L Power Stroke caused us to take a different look at fuel and how much of a concern it really is. No longer could we consider 2.0% is a major problem. Now we suggest that it’s only an issue if the oil level is rising on your dipstick, or if the amount of fuel we find in each sample is increasing. As it turns out, continual fuel dilution in the oil at around 2.0% to 3.0% sometimes is from a problem, but it should not be considered a major one and I know about that first-hand.
About my Passat
In 2004 my wife and I bought a Volkswagen Passat with the 1.8L turbo gasoline engine. Almost from the start, this engine was leaving a lot of fuel in the oil and I would look at the analysis results and just shrug my shoulders. The engine was running fine and wear metals were acceptable, but the fuel mileage was never quite a good as advertised. For me, that didn’t seem like a good enough reason to tear into the fuel system.
Shortly after we bought the Passat, Volkswagen set us a letter saying they would extend the engine warranty to 10 years or 100,000 miles due to sludging problems they were having. I suspected these problems stemmed from a lot of fuel dilution in the oil coupled with really long oil runs, but I’m not sure. The kicker for the extend warranty was I had to change oil every 5,000 miles and I had to use a VW-approved oil. Of course, they approved expensive oils like Elf and Total, and those aren’t on my approved list. My list includes oils that are on sale at Wal-Mart, so I decided to stick with my oils and just change the oil at 3,000 miles. So far the plan has worked but if it fails, I’ll be writing about how I rebuilt the engine myself (twice) in my Dad’s barn.
In the end, we haven’t done anything about the continual fuel in our Passat’s oil (except curse VW), but the engine is still running fine and is close to the magic 100,000-mile mark. When we hit 100K, we’ll unload it and get my wife the new car of her dreams (a white Jaguar S-type). So despite the fuel being present in every report, really the only problem this has caused is our MPG isn’t quite what it should be.
Back to diesel engines
So anyway, the fuel dilution problems in the 6.0L Power Stroke eventually got better and those engines now look as good as any we see, so they’ve changed something to solve the fuel problem.
Then came the next generation of diesels (the 6.4L Power Stroke) and the fuel problems started up again. It’s not uncommon to sees excess fuel in over 2% of the small diesel engine samples we see today, and when it shows up that often, it’s hard to say it’s a major issue. It shouldn’t really be there, but it doesn’t necessarily warrant a trip to the dealer either.
The source of the fuel dilution differs from one engine manufacturer to the next, though injectors and emission control systems appear to be the root cause of most of these problems.
For the new 6.4L Power Strokes, if it’s not an injector it could be another part of the fuel system, like a pump. The DPF (diesel particulate filter) regeneration process will also cause fuel to show up in the oil. Does that mean these new engines are junk? Not at all. It just shows they have some growing pains to work out and once that happens, the fuel dilution problems will eventually taper off.
Until then, don’t get too excited 2.0% or more of fuel dilution, but do watch for an increased oil level on your dipstick. While you may think an engine that makes oil is like the goose that laid the golden egg, it’s really a possible sign of problems down the road. Small amounts of fuel are okay, but if the oil level is rising or if we’re seeing more and more fuel in each sample you do, fuel could be a problem.
Fuel Contamination in Aircraft
Here in the northern latitudes autumn brings uncertainty about what to expect from the sky and wind each morning. Rain and overcast skies are frequent but counterbalanced by days when clear blue skies are accented with yellow sunlight that reflects the fall leaves and warms the spirit.
Those who fly in the winter months generally count the experience with mixed feelings. Cold toes and fingers are a certainty. So are hard-to-start engines and batteries that lack enthusiasm. But once the engine finally fires and the first BTUs of heat start filtering into the frosty cabin air, the whole experience can bring a smile to the face of the most determined pessimist.
The brakes may be stiff but they still work. Once you get to the run-up area, most of the breath-laden frost has cleared from the windscreen and you can stow the gloves. The sun streaming into the cabin does as much to warm things as the manifold heater. On lift-off, the rewards of winter flying come back to remind you why you do this in the first place. The prop bites the crisp air with authority. The dense air brings lift with a rush. Like the counter guy at the FBO said, “There’s a lot of lift going on out there today!” Indeed!
Understanding the gas
There are several reasons engines are hard to start in the cold. Parts are machined to operate easily in concert when they are at operating temperature. The further you get from that temperature—either hot or cold—the more interference there will be between interfacing parts. Poorer fitting parts increase internal engine friction.
Air-cooled aircraft engines typically run on SAE 50W oil when at operating temperature. Cold, the oil has the properties of molasses. The oil pump resists rotation. The oil resists being pushed around. The oil that starts out in the oil cooler may still be there when you land. If the surface air is cold, you know the air at altitude will be colder. It is not unusual to find the oil temperature at the bottom of the green arc, if it gets into the green at all. Pity the poor battery that has to coax all this reluctance into motion.
Mixture matters
Another reason cold engines are hard to start is the gas/air mixture is incorrect. The fuel system, whether carbureted or injected, is set up to operate at a given mixture for normal temperature operation, usually fifteen or sixteen parts air to one part gas. Cold cylinder walls condense gas from the mixture, causing the gas that’s left to become lean—far too lean to initiate normal combustion. Accordingly, engines of all types have an enrichment device to compensate when cold. For liquid-cooled land-based engines, a choke in the carb throat or an extra injector enriches the intake air. Both types of systems usually shut off automatically as the cylinder walls warm up, which usually doesn’t take long.
Air-cooled aircraft engines, on the other hand, have a primer. Even when carbureted, they won’t use chokes or have the acceleration pumps that are common for car and truck engines. Many aircraft engines that fire readily on zero or minimal prime on a sunny, warm day won’t even consider firing without prime in winter. If one squirt of prime works in July, it may take four to six in January.
If there is moisture in the first gasp of cold air that gets sucked into the cylinders, it can frost the plug electrodes. If this happens, no amount of priming or cranking (or swearing) will make any difference. The engine won’t fire until the frost is melted or otherwise eliminated.
Raw gas that condenses on the cold cylinder walls gets scraped down into the oil by the beveled oil control ring(s). It will mix perfectly with oil, so there is no good way to get it back out again unless you cook it out with heat and agitation (otherwise known as flying). If it is cold enough at the altitudes you fly, the gas from priming may still be in the oil when you land.
Problem or not?
But does gas in the oil really hurt anything? Hardly. It will cause a lower viscosity, but that may be an asset rather than a problem. There were WWII radial engines operating in the frozen north that were designed to inject gas into the oil before shutting down. The gas thinned the oil so that the engine could be cranked over in the morning with less resistance. After the engine got back to operating temperature, the oil returned to being an SAE 50 or 60W once the gas was distilled back out of the oil…sort of an automatic multi-weight oil before multi-weight oils were invented.
To say we find a lot of gas contamination in winter oil samples would be an understatement. We usually mention it because gas in the oil can show a fuel system problem. But that is rare in aircraft engines. We find a lot of moisture in winter oil samples too, and it goes into the same category as the fuel. Unless your aircraft engine is liquid-cooled, we don’t think the moisture is any more a problem than the gas.
When taking your sample, it’s ideal to have the oil warmed to operating temperature first, though if that’s not possible it’s best to just take the sample cold and not start the engine at all. Starting the engine but not flying it can introduce even more gas into the system. We realize an FBO mechanic won’t have the option of taking your airplane for a couple of turns around the pattern, even if he or she was qualified to do so, before draining the oil. Consequently, we turn up volatile gas and often moisture in your winter samples if you are flying in the cold. It only rarely points to a problem.
What is a Flashpoint?
We use the flashpoint test to determine how much fuel dilution is present in your oil. Technically speaking, the flashpoint is the lowest temperature at which a liquid will generate sufficient vapor to flash (ignite) when exposed to a source of ignition or fire. In other words, at what temperature do the vapors coming off your oil catch fire? For most gasoline oil samples, it’s around 380°F. For most diesel samples, it’s about 410°F.
Each brand/type of oil has an expected “should be” value for the flashpoint, and when the lab test results read lower than that value, it shows a contaminant in the oil. Most often that contaminant is fuel, though other things can affect the flashpoint too, such as solvents (like engine cleaner additives) or water. We calculate the amount of fuel present based on where the flashpoint is relative to the “should be” value and the volatility of the type of fuel you’re using in the engine. Alternative fuels like B20 can have a different impact on the flashpoint than standard fuels, so be sure to let us know if you’re using anything other than standard gas/diesel as fuel in your engine.
Based on the margin of error for the methodology we use for measuring the flashpoint, the lowest fuel dilution value you’ll see on one of our reports is <0.5%. That’s our way of essentially saying that no measurable fuel dilution was detected in the oil. If the flashpoint of your sample reads the same as the “should be” value, we’ll report a “TR” (or trace) of fuel dilution. In other words, it’s likely there was a very small amount of fuel dilution present, but not enough to quantify. After that, you’ll see fuel dilution reported as a percentage of the sample. The most fuel our test can accurately read is 10%. If you have more than that, we’ll report >10% (and you should head to a mechanic).
How much fuel is too much? It depends. We have different allowances for different types of engines based on their typical operational conditions, and we share those values in the “should be” column. If you’re constantly exceeding those values, you might consider the type of operation the engine sees just before sampling. Are you idling the engine to warm it up? Have you just been running errands around town? Is the dealer changing your oil (and starting your engine briefly to pull the vehicle onto a lift)? That type of operation can introduce a little fuel dilution into the oil and as such isn’t necessarily a concern. If the amount of fuel in the oil is consistently above 2.0-3.0%, or if it’s increasing from sample to sample, that might indicate a more serious problem.
A little fuel dilution – the type you’d get in your oil from operational factors — will cook out of the oil once the oil reaches operational temperature. If there’s a fuel dilution problem, though, you’ll see telltale signs: a rising oil level, high fuel dilution readings in testing, a strong fuel smell to the oil, and possibly low viscosity readings and increasing wear as well. The concern with excessive fuel dilution is that it dilutes and thins the oil, which might limit the oil’s ability to effectively protect and cool your engine.